Abstract
Purpose
To test whether co-delivery of anticancer small interfering RNA (siRNA) and a chemical MEK inhibitor using cationic liposomes enhances anticancer activity in vitro and in vivo.
Method
MEK inhibitor PD0325901 was encapsulated in lipid layers of N',N''-dioleylglutamide-based cationic liposomes (DGL). Mcl1-specific siRNA (siMcl1) was complexed to DGL or PD0325901-loaded liposomes (PDGL). Efficiency of cellular siRNA delivery was tested using fluorescent double-stranded RNA. Silencing of target proteins was evaluated using Western blotting and real-time quantitative polymerase chain reactions. In vivo anticancer activity was tested using xenografted mice.
Results
Size and zeta potential of PDGL were similar to DGL. PDGL could deliver double-stranded RNA into cells with efficiencies comparable to DGL. Cellular co-delivery of siMcl1 and PD0325901 reduced expression of Mcl1 and pERK1/2 proteins and more effectively reduced tumor cell survival than other treatments. In mice, siMcl1 and PD0325901 co-delivered by PDGL inhibited growth of tumors 79%. Substantial apoptosis of tumor cells was observed following PDGL-mediated co-delivery of siMcl1, but not in other groups.
Conclusions
PDGL-mediated co-delivery of siMcl1 and MEK inhibitor, PD0325901, could serve as a potential strategy for combination chemogene anticancer therapy.






Similar content being viewed by others
Abbreviations
- DG:
-
N’,N”-dioleylglutamide
- DGL:
-
DG-based cationic liposomes
- DOPE:
-
dioleyl-sn-glycero-3-phosphoethanolamine
- dsRNA:
-
double-stranded RNA
- ERK:
-
extracellular signal-related kinase
- GAPDH:
-
glyceraldehyde-3-phosphate dehydrogenase
- L2K:
-
Lipofectamine 2000
- Mcl1:
-
myeloid cell leukemia sequence 1
- MEK:
-
mitogen-activated protein/extracellular signal-regulated kinase kinase
- MTT:
-
3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide
- PCR:
-
polymerase chain reaction
- PDGL:
-
PD0325901-loaded cationic liposomes
- pERK1/2:
-
phospho-ERK1/2
- siGL2:
-
luciferase-specific siRNA
- siMcl1:
-
Mcl1-specific siRNA
- siRNA:
-
small interfering RNA
REFERENCES
Kato T, Natsume A, Toda H, Iwamizu H, Sugita T, Hachisu R, et al. Efficient delivery of liposome-mediated MGMT-siRNA reinforces the cytotoxity of temozolomide in GBM-initiating cells. Gene Ther. 2010;17:1363–72.
Simonin K, Brotin E, Dufort S, Dutoit S, Goux D, N'diaye M, et al. Mcl-1 is an important determinant of the apoptotic response to the BH3-mimetic molecule HA14-1 in cisplatin-resistant ovarian carcinoma cells. Mol Cancer Ther. 2009;8:3162–70.
Singh A, Boldin-Adamsky S, Thimmulappa RK, Rath SK, Ashush H, Coulter J, et al. RNAi-mediated silencing of nuclear factor erythroid-2-related factor 2 gene expression in non-small cell lung cancer inhibits tumor growth and increases efficacy of chemotherapy. Cancer Res. 2008;68:7975–84.
Patil YB, Swaminathan SK, Sadhukha T, Ma L, Panyam J. The use of nanoparticle-mediated targeted gene silencing and drug delivery to overcome tumor drug resistance. Biomaterials. 2010;31:358–65.
Chen AM, Zhang M, Wei D, Stueber D, Taratula O, Minko T, et al. Co-delivery of doxorubicin and Bcl-2 siRNA by mesoporous silica nanoparticles enhances the efficacy of chemotherapy in multidrug-resistant cancer cells. Small. 2009;5:2673–7.
Meng H, Liong M, Xia T, Li Z, Ji Z, Zink JI, et al. Engineered design of mesoporous silica nanoparticles to deliver doxorubicin and P-glycoprotein siRNA to overcome drug resistance in a cancer cell line. ACS Nano. 2010;4:4539–50.
Chen Y, Bathula SR, Li J, Huang L. Multifunctional nanoparticles delivering small interfering RNA and doxorubicin overcome drug resistance in cancer. J Biol Chem. 2010;285:22639–50.
Azmi AS, Wang Z, Philip PA, Mohammad RM, Sarkar FH. Proof of concept: network and systems biology approaches aid in the discovery of potent anticancer drug combinations. Mol Cancer Ther. 2010;9:3137–44.
Wang D, Boerner SA, Winkler JD, LoRusso PM. Clinical experience of MEK inhibitors in cancer therapy. Biochim Biophys Acta: Mol Cell Res. 2007;1773:1248–55.
Haura EB, Ricart AD, Larson TG, Stella PJ, Bazhenova L, Miller VA, et al. A phase II study of PD-0325901, an oral MEK inhibitor, in previously treated patients with advanced non-small cell lung cancer. Clin Cancer Res. 2010;16:2450–7.
Ciuffreda L, Del Bufalo D, Desideri M, Di Sanza C, Stoppacciaro A, Ricciardi MR, et al. Growth-inhibitory and antiangiogenic activity of the MEK inhibitor PD0325901 in malignant melanoma with or without BRAF mutations. Neoplasia. 2009;11:720–31.
McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–84.
Rinehart J, Adjei AA, Lorusso PM, Waterhouse D, Hecht JR, Natale RB, et al. Multicenter phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, and pancreatic cancer. J Clin Oncol. 2004;22:4456–62.
Henderson YC, Chen Y, Frederick MJ, Lai SY, Clayman GL. MEK inhibitor PD0325901 significantly reduces the growth of papillary thyroid carcinoma cells in vitro and in vivo. Mol Cancer Ther. 2010;9:1968–76.
Kinkade CW, Castillo-Martin M, Puzio-Kuter A, Yan J, Foster TH, Gao H, et al. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J Clin Invest. 2008;118:3051–64.
Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–37.
Song L, Coppola D, Livingston S, Cress D, Haura EB. Mcl-1 regulates survival and sensitivity to diverse apoptotic stimuli in human non-small cell lung cancer cells. Cancer Biol Ther. 2005;4:267–76.
Wei S-H, Dong K, Lin F, Wang X, Li B, Shen JJ, et al. Inducing apoptosis and enhancing chemosensitivity to Gemcitabine via RNA interference targeting Mcl-1 gene in pancreatic carcinoma cell. Cancer Chemo Pharmacol. 2008;62:1055–64.
Oh YK, Park TG. siRNA delivery systems for cancer treatment. Adv Drug Deliv Rev. 2009;61:850–62.
Kim WJ, Kim SW. Efficient siRNA delivery with non-viral polymeric vehicles. Pharm Res. 2009;26:657–66.
Nguyen J, Reul R, Roesler S, Dayyoub E, Schmehl T, Gessler T, et al. Amine-modified poly(vinyl alcohol)s as non-viral vectors for siRNA delivery: effects of the degree of amine substitution on physicochemical properties and knockdown efficiency. Pharm Res. 2010;27:2670–82.
Suh MS, Shim G, Lee HY, Han S-E, Yu Y-H, Choi Y, et al. Anionic amino acid-derived cationic lipid for siRNA delivery. J Control Release. 2009;140:268–76.
Oh YK, Suh D, Kim JM, Choi HG, Shin K, Ko JJ. Polyethylenimine-mediated cellular uptake, nucleus trafficking and expression of cytokine plasmid DNA. Gene Ther. 2002;9:1627–32.
Mao S, Sun W, Kissel T. Chitosan-based formulations for delivery of DNA and siRNA. Adv Drug Deliv Rev. 2010;62:12–27.
Wei SH, Dong K, Lin F, Wang X, Li B, Shen JJ, et al. Inducing apoptosis and enhancing chemosensitivity to gemcitabine via RNA interference targeting Mcl-1 gene in pancreatic carcinoma cell. Cancer Chemother Pharmacol. 2008;62:1055–64.
Del Prete MJ, Robles MS, Guáo A, Martínez-A C, Izquierdo M, Garcia-Sanz JA. Degradation of cellular mRNA is a general early apoptosis-induced event. FASEB J. 2002;16:2003–5.
Sun TM, Du JZ, Yao YD, Mao CQ, Dou S, Huang SY, et al. Simultaneous delivery of siRNA and paclitaxel via a “two-in-one” micelleplex promotes synergistic tumor suppression. ACS Nano. 2011;5:1483–94.
Cao N, Cheng D, Zou S, Ai H, Gao J, Shuai X. The synergistic effect of hierarchical assemblies of siRNA and chemotherapeutic drugs co-delivered into hepatic cancer cells. Biomaterials. 2011;32:2222–32.
Zhu C, Jung S, Luo S, Meng F, Zhu X, Park TG, et al. Co-delivery of siRNA and paclitaxel into cancer cells by biodegradable cationic micelles based on PDMAEMA-PCL-PDMAEMA triblock copolymers. Biomaterials. 2010;31:2408–16.
Brown AP, Carlson TC, Loi CM, Graziano MJ. Pharmacodynamic and toxicokinetic evaluation of the novel MEK inhibitor, PD0325901, in the rat following oral and intravenous administration. Cancer Chemother Pharmacol. 2007;59:671–9.
Brown AP. Development of serum calcium and phosphorus as clinical biomarkers for drug-induced systemic mineralization: case study with a MEK inhibitor. In: Bleavins MR, Carini C, Jurima-Romet M, Rahbari R, editors. Biomarkers in drug development: a handbook of practice, application, and strategy. Hoboken: John Wiley & Sons; 2010. p. 301–22.
ACKNOWLEDGMENTS & DISCLOSURES
This study was financially supported by a grant of the Korean Health Technology R&D Project (Grant No. A090945), Ministry for Health, Welfare & Family Affairs, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Seung Hee Kang and Hee-Jeong Cho contributed equally to this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig S1
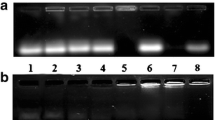
Effect of N/P ratios on cellular uptake of fluorescent dsRNA/PDGL complexes. (PPT 364 kb)
Rights and permissions
About this article
Cite this article
Kang, S.H., Cho, HJ., Shim, G. et al. Cationic Liposomal Co-delivery of Small Interfering RNA and a MEK Inhibitor for Enhanced Anticancer Efficacy. Pharm Res 28, 3069–3078 (2011). https://doi.org/10.1007/s11095-011-0569-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-011-0569-4